ICH M4 各模块思维导图
CTD Triangle
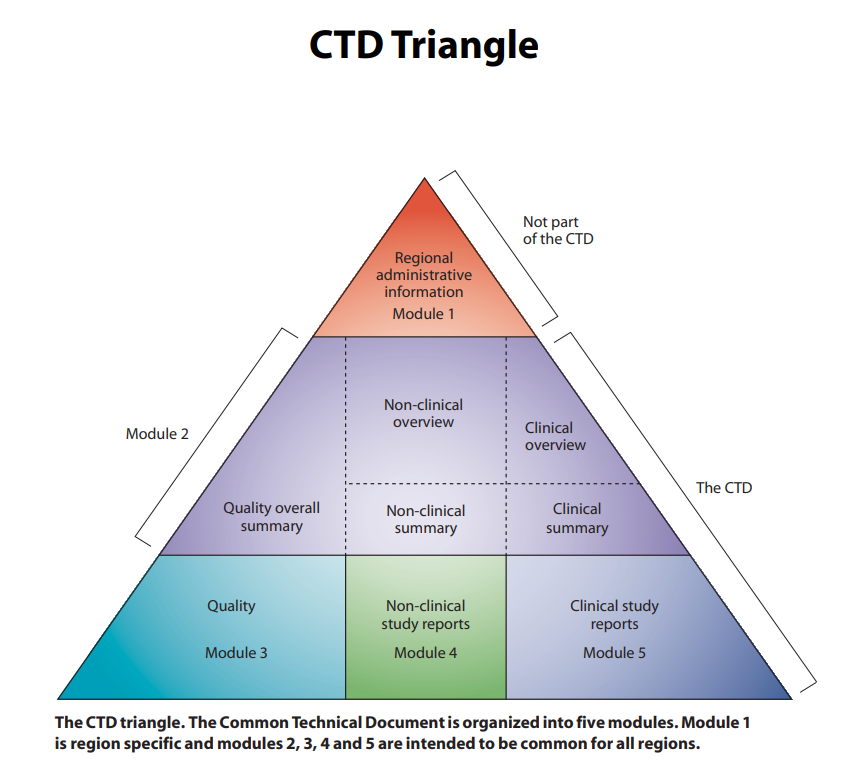
Figure 1. CTD triangle
ICH M4 各模块思维导图
注:图片如果不清晰可以在页面底部下载源文件查看。
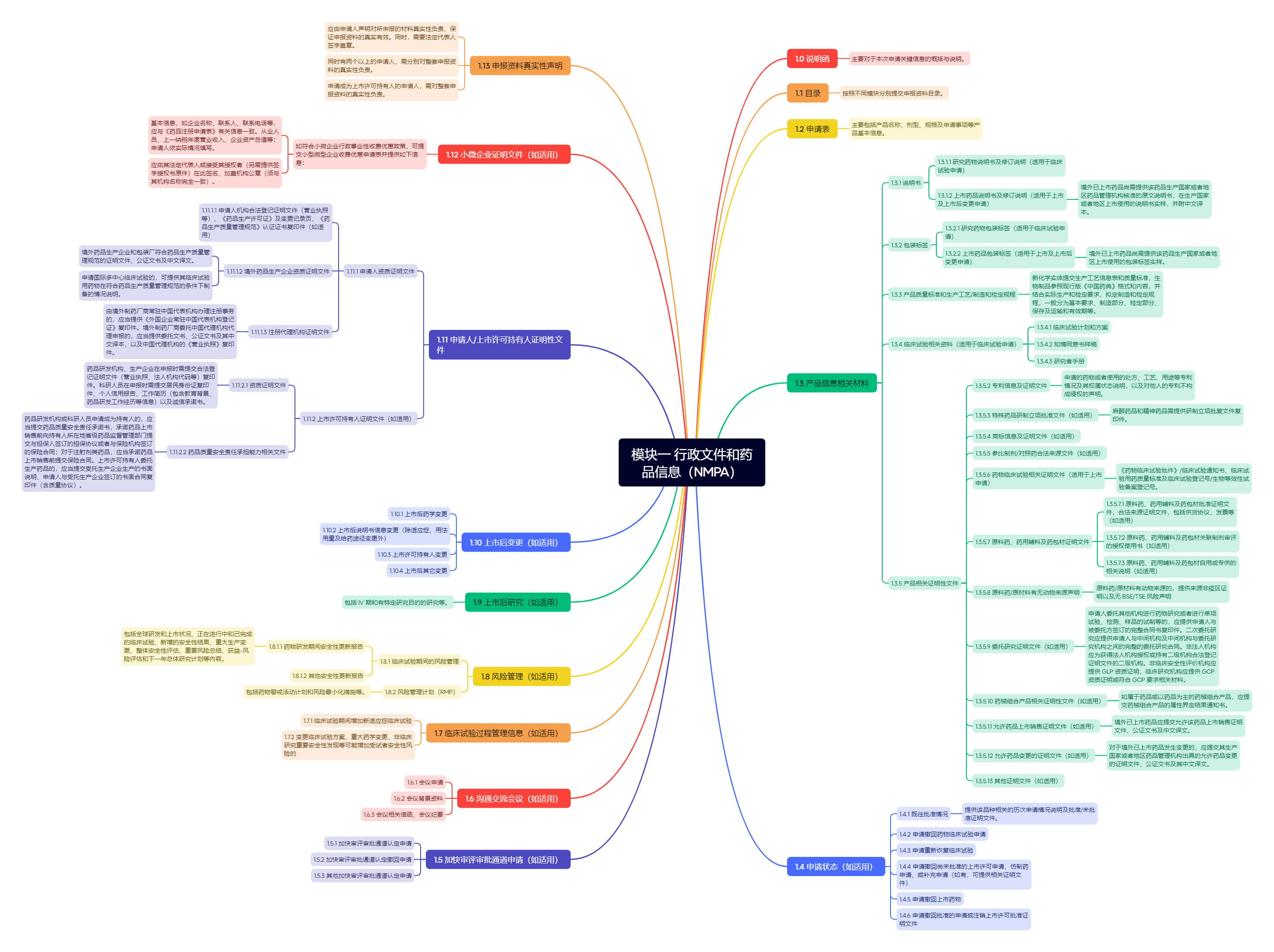
Figure 2. 模块一:行政文件和药品信息(NMPA)
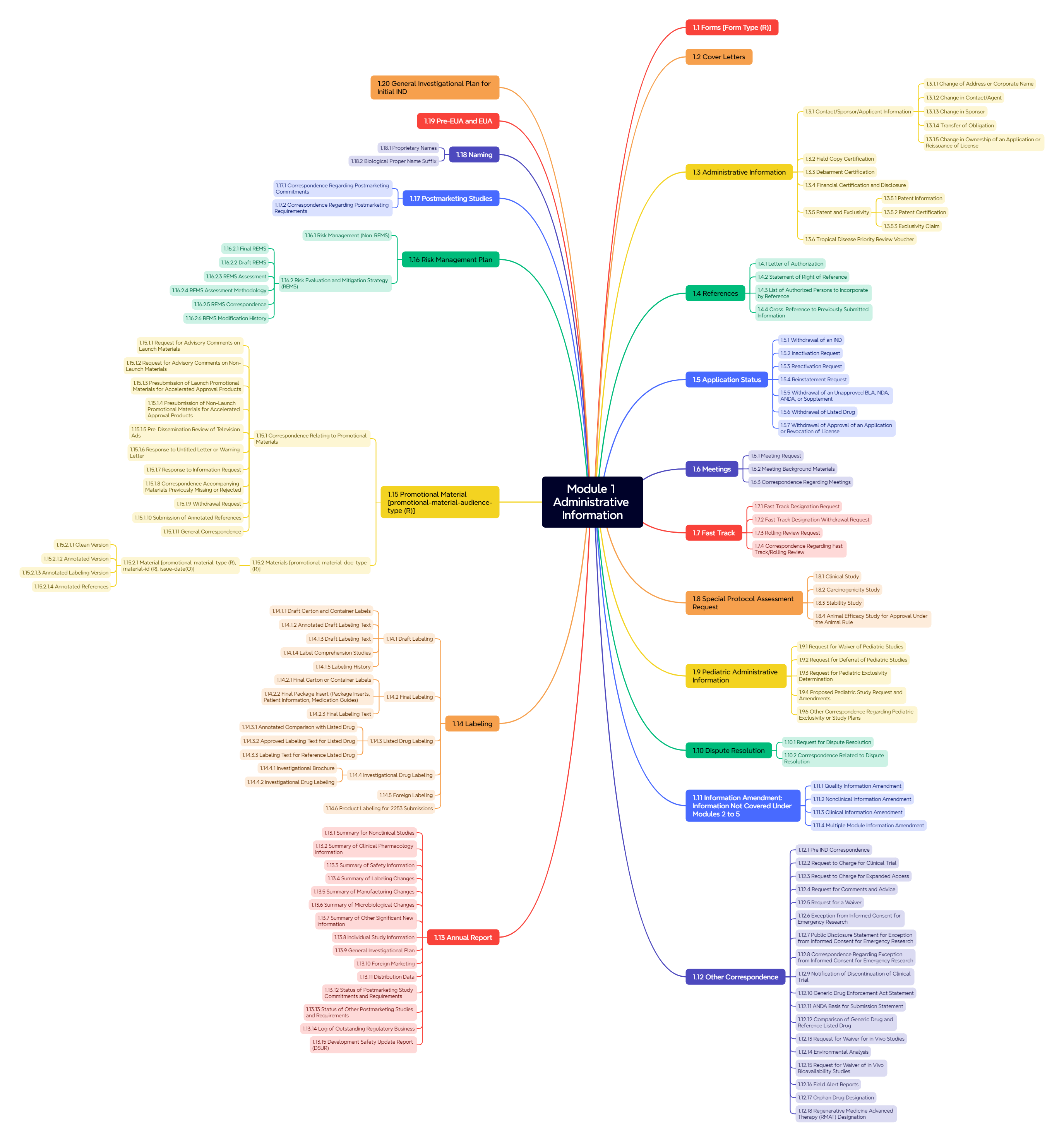
Figure 3. Module 1: Administrative Information (FDA)
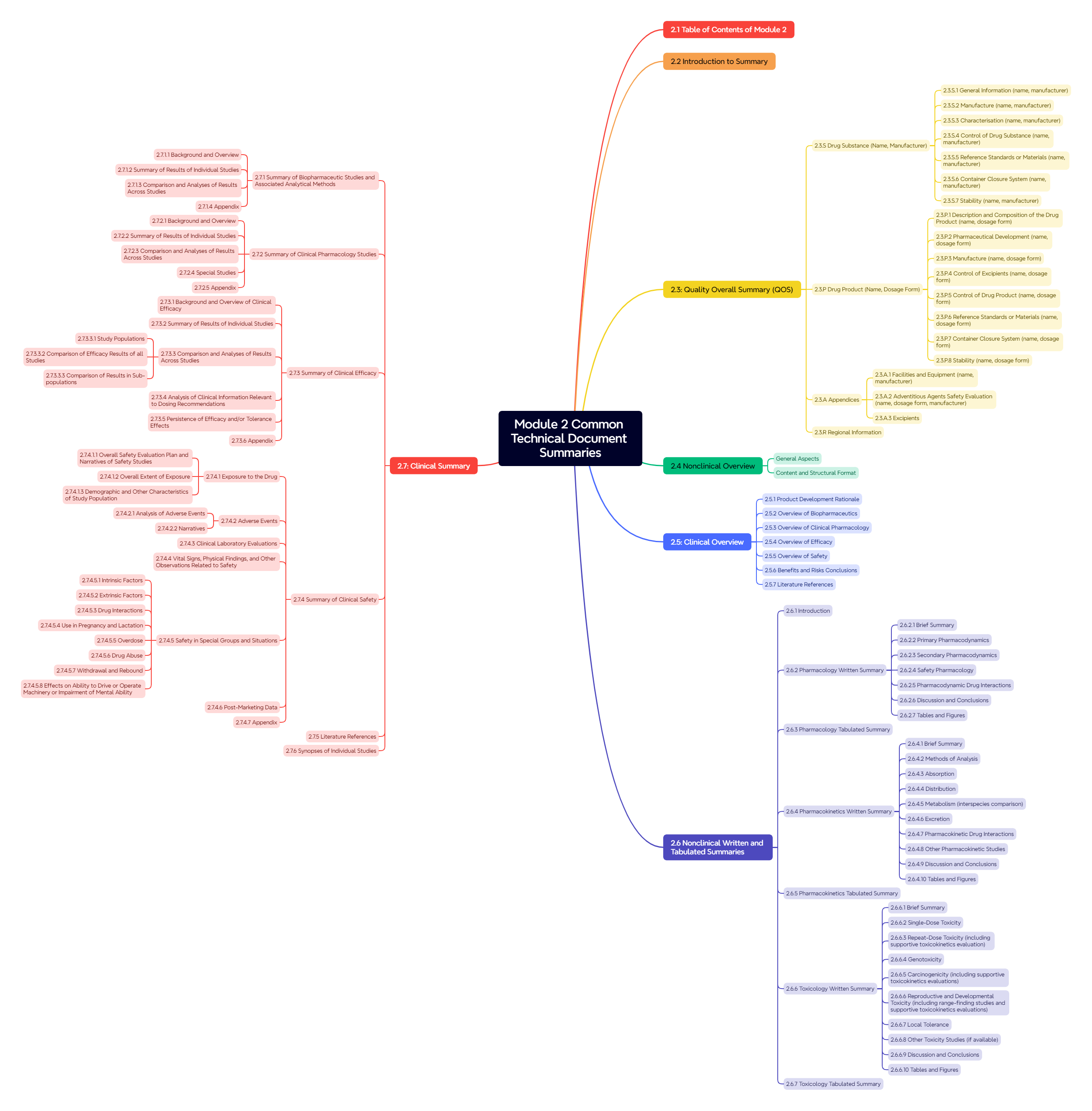
Figure 4. Module 2: Common Technical Document Summaries
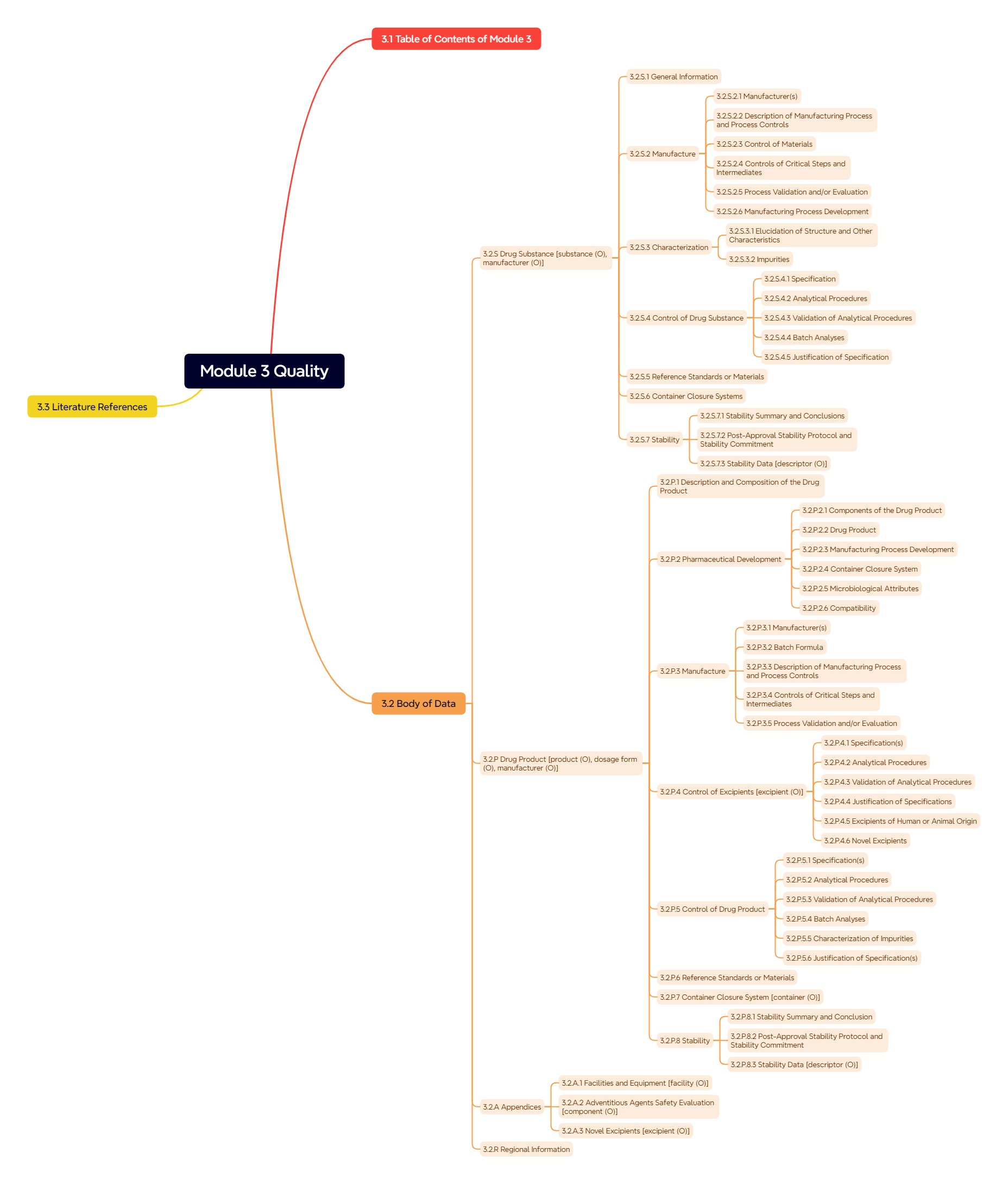
Figure 5. Module 3: Quality
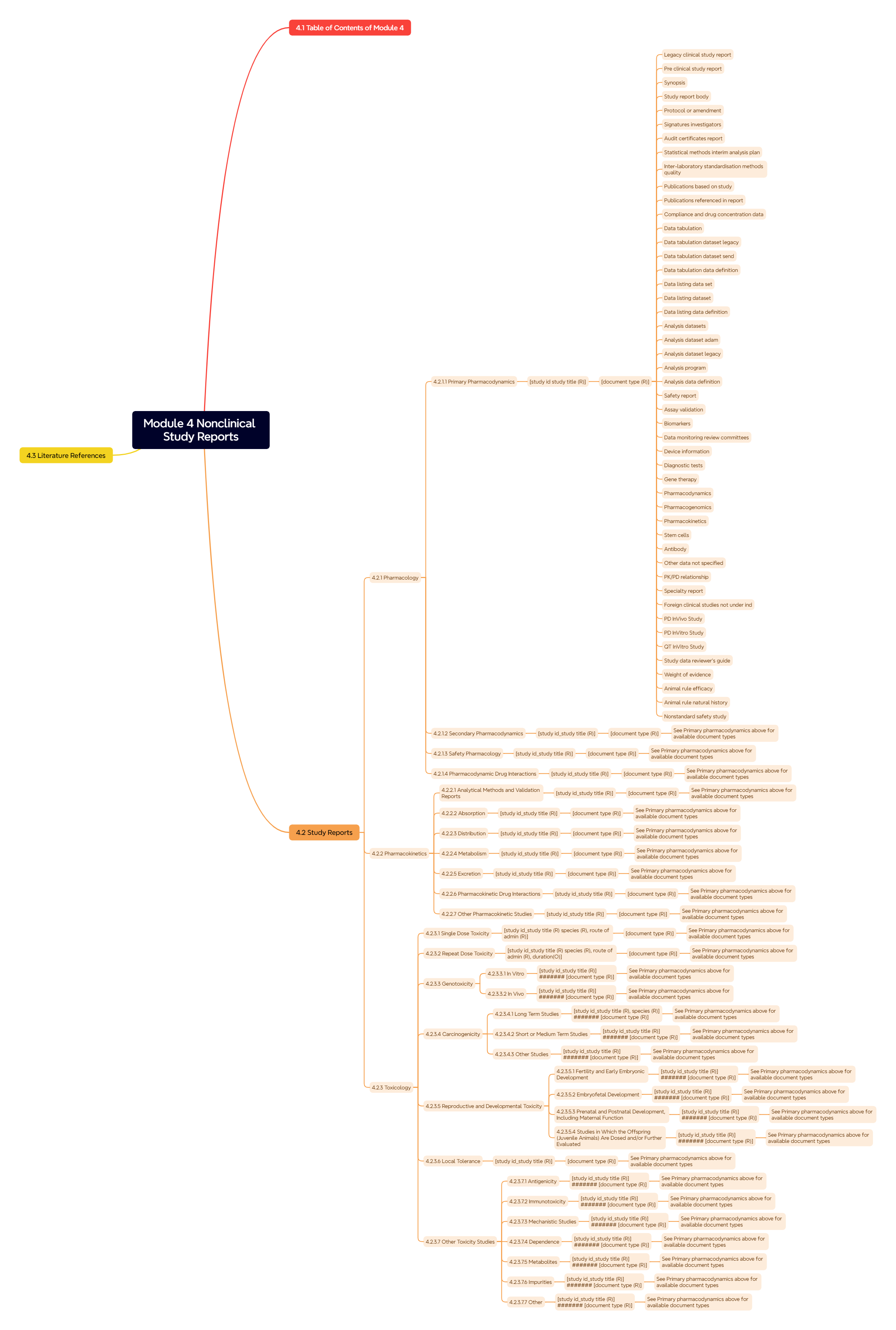
Figure 6. Module 4: Nonclinical Study Reports
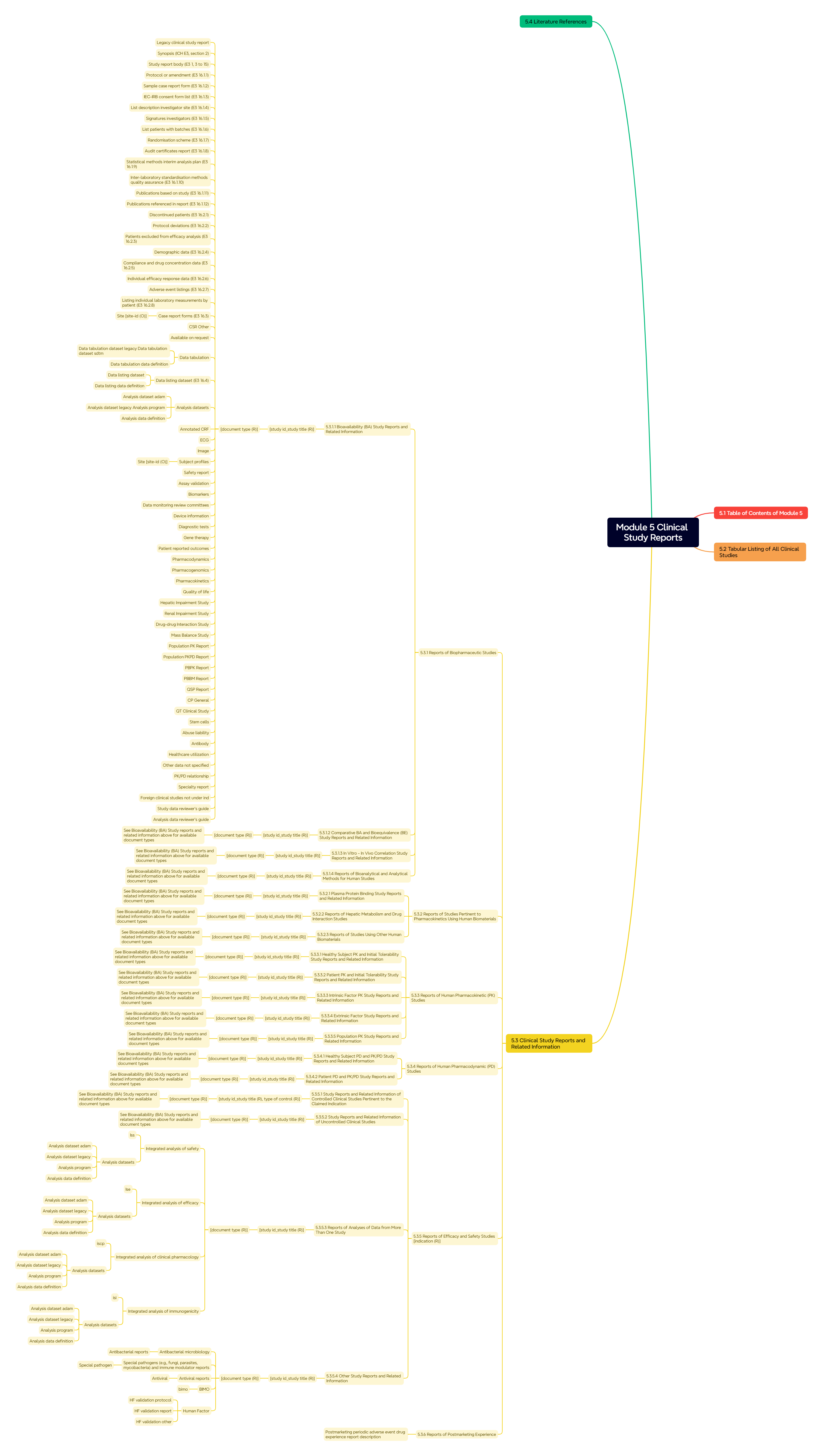
Figure 7. Module 5: Clinical Study Reports
Legend:
- (R)—Required
- (O)—Optional
源文件下载
在线思维导图
模块一:行政文件和药品信息(NMPA)
http://naotu.baidu.com/file/0768de540b954c1283c477b28b55a5aa?token=f1691d1f702c225c
Module 1: Administrative Information (FDA)
http://naotu.baidu.com/file/4c9822871273da1faf654c966ae64394?token=2086201a41826291
Module 2: Common Technical Document Summaries
http://naotu.baidu.com/file/b5e7a6007e9e1b2f398d848a377aa9cf?token=3ddb86019834e0c8
Module 3: Quality
http://naotu.baidu.com/file/4818489411d37186d7e8ebd282938d8d?token=91f7577e4fbf8b27
Module 4: Nonclinical Study Reports
http://naotu.baidu.com/file/a5008ae1e53d4de59bd3e4dd9dca7262?token=7154cf7d2329de87
Module 5: Clinical Study Reports
http://naotu.baidu.com/file/7ad0821db331dfd4c62aac15d4a79081?token=df5ab4150a74e6e4
文件下载
Markdown: ICH_M4_Markdown.zip
Xmind: ICH_M4_Xmind.zip
References
ICH CTD: https://www.ich.org/page/ctd
国家药监局关于发布《M4:人用药物注册申请通用技术文档(CTD)》模块一文件及CTD中文版的通告(2019年第17号):https://www.nmpa.gov.cn/directory/web/nmpa/xxgk/ggtg/ypggtg/ypqtggtg/20190417174001488.html
eCTD v4.0 Comprehensive Table of Contents Headings and Hierarchy: https://www.fda.gov/media/179699/download?attachment